Which of the Following Gases Deviates Most From Ideal Behavior
Also gases are more likely to be ideal at temperatures well above the liquefying point. Mechanical Engineering questions and answers.

Solved Questions At 320 K And 5 Mpa Which Of The Following Chegg Com
The stronger the intermolecular attractions in a gas sample the less ideal the gas is.

. The ideal gas composed of more than one atom is hydrogen gas. Under the same conditions which of the following gases deviates most from ideal from CHEMISTRY 61 at Applied Technology High School Abu Dhabi Boys Campus. Why does carbon dioxide deviate from ideal gas law.
Also this same gas has higher MP and BP than the other gases and significantly closer to room temperature. Many gases such as nitrogen oxygen hydrogen noble gases and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Which of the following is one reason for this difference.
HCl consists of polar molecules that are held together by the stronger dipole-dipole interactions. Because their particles occupy finite space and do exert interactive forces among themselves. Hence they deviate from ideal behaviour.
N2 O2 and CH4 are all non-polar molecules with London dispersion forces the weakest form of intermolecular attractions. Solve any question of States Of Matter with-. The combined volume of the Ar atoms is too large to be negligible compared with the total volume of the container.
Why real gases deaviat from ideal behaviou. The electrical charge is spread across two atoms. Many gases such as nitrogen oxygen hydrogen noble gases and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances.
Highly polar gases deviate from ideal behavior such as ammonia NH3 and water vapor H2O to a larger degree than polar gases. Lower density gases are more likely to behave as ideal gases. Its a question intermolecular forces both attractive and repulsive.
Deviation from ideality can be desctibed by the compressibility factor. In most usual conditions for instance at standard temperature and pressure most real gases behave qualitatively like an ideal gas. Ar g deviates more from ideal behavior at extremely high pressures than Ne g does.
Solution for Which of the following gases deviates the most from ideal behavior. Solution for Which of the following gases deviates the most from ideal behavior. The study of respondent behavior is to ________ as the study of operant behavior is to ________.
PUH Save Ans Knowing the compressibility factor of the following gases which. CO2 N2 H2S F2. Like a helium atom a hydrogen molecule also has two electrons and its intermolecular forces are small.
Real gases deviate from Ideal gases. Also the intermolecular forces start acting upon the molecules. Answer 1 of 3.
In most usual conditions for instance at standard temperature and pressure most real gases behave qualitatively like an ideal gas. As gas molecules get larger they behave less like ideal gases. P V nb nRT P V n b n R T.
PUH Save Ans Knowing the compressibility factor of the following gases which gass behavior below deviates the most from ideal gas behavior. This makes it less ideal. Perfect ideal gas is a hypothetical case because at low temperature and high pressure real gases.
Gas E Z12 Gas C Z1 Gas B Z02 O Gas D Z08 O Gas A Z15. As all gases have the same volume the gas with the greater molar mass will have the higher density. Dispersion forces increase and dipole-dipole interaction may occur.
Which of the following gases will effuse the most rapidly. The van der Waals equation modifies the ideal gas law to correct for this excluded volume and is written as follows. Many gases begin to deviate from ideal behavior under which of the following conditions.
An ideal gas differs from a real gas in that the molecules of an ideal gas _____. At high pressures the deviation from ideal behavior occurs because the finite volume that the gas molecules occupy is significant compared to the total volume of the container. CO 2 N2 H 2 S F2.
Answer 1 of 7. The molecular volume also plays a role but mainly at high pressure high density. The particle volume of ArAr is greater than that of NeNe.
560 students attemted this question. At low temperature and high pressure the volume of the particles is not negligible as compared to the volume of the gas. Here are the graphs containing ammonia and.
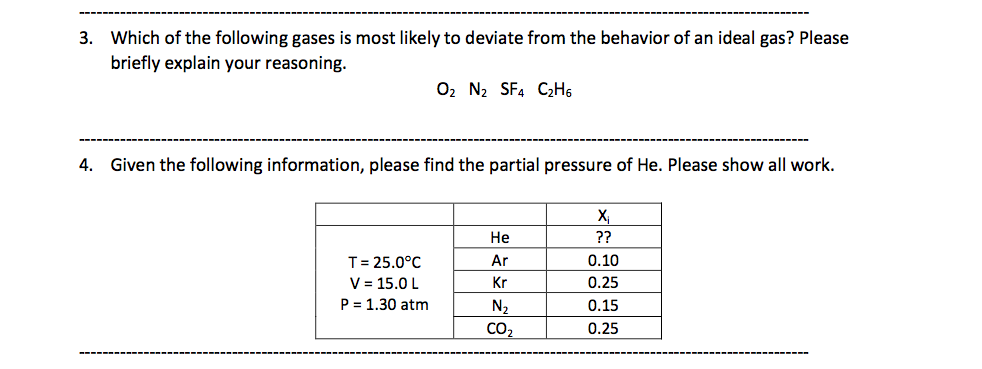
Solved 3 Which Of The Following Gases Is Most Likely To Chegg Com
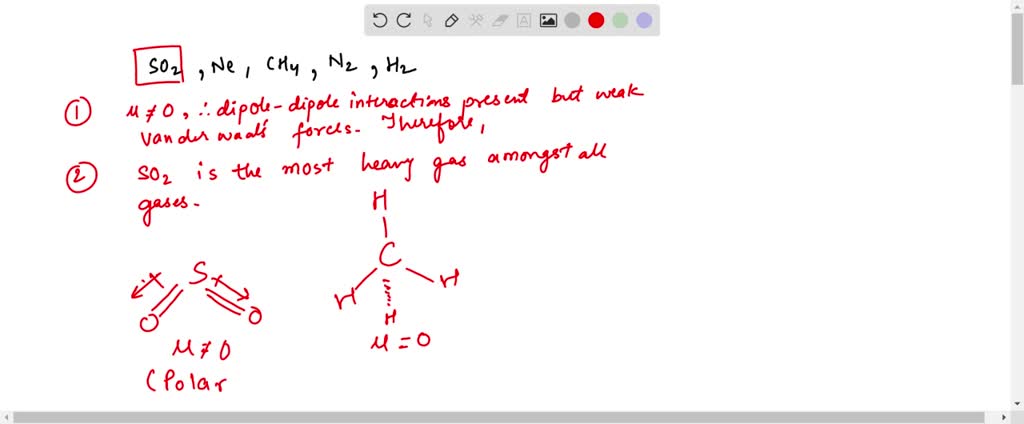
Solved Which Of The Following Gases Deviates Most From Ideal Behavior Justify Your Answer With At Least Two Reasons So2 Ne Ch4 N2 H2

Solved Which Of The Following Gases Deviates Most From Ideal Chegg Com
No comments for "Which of the Following Gases Deviates Most From Ideal Behavior"
Post a Comment